When we talk about chemical reaction as balanced, we mean that both sides of the given equation (reactants and products) need to have the same number of molecules for each of the elements.
In our case, photosynthesis is the conversion of carbon dioxide (taken in by the plants) and water (taken from the roots) to synthesize nutrition (energy) in the presence of sunlight, whilst releasing oxygen as a byproduct.
Balanced Equation Of Photosynthesis Is Given As Below:
6 CO2 + 6 H2O → C6H12O6 + 6 O2
Graphically shown:
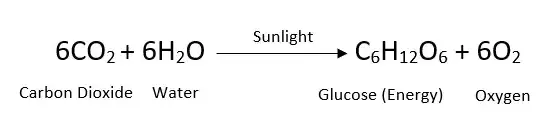 Where:
Where:
- CO2 = carbon dioxide
- H2O = water
- light is required
- C6H12O6 = glucose
- O2 = oxygen
Scientists further explain this equation as 6 molecules of carbon dioxide reacting with 6 molecules of water in the presence of sunlight to produce 1 molecule of glucose and 6 molecules of oxygen.
Note:
Sunlight is necessary as the photons provide the energy for the reaction to take place.



